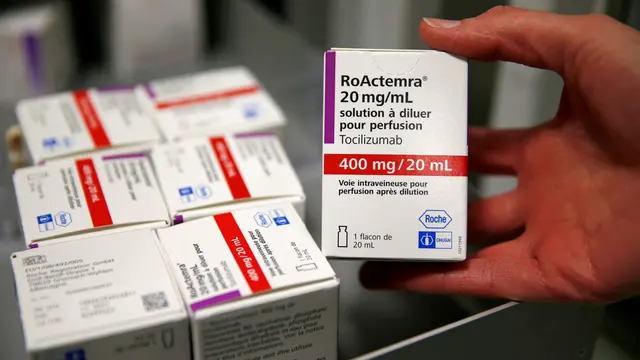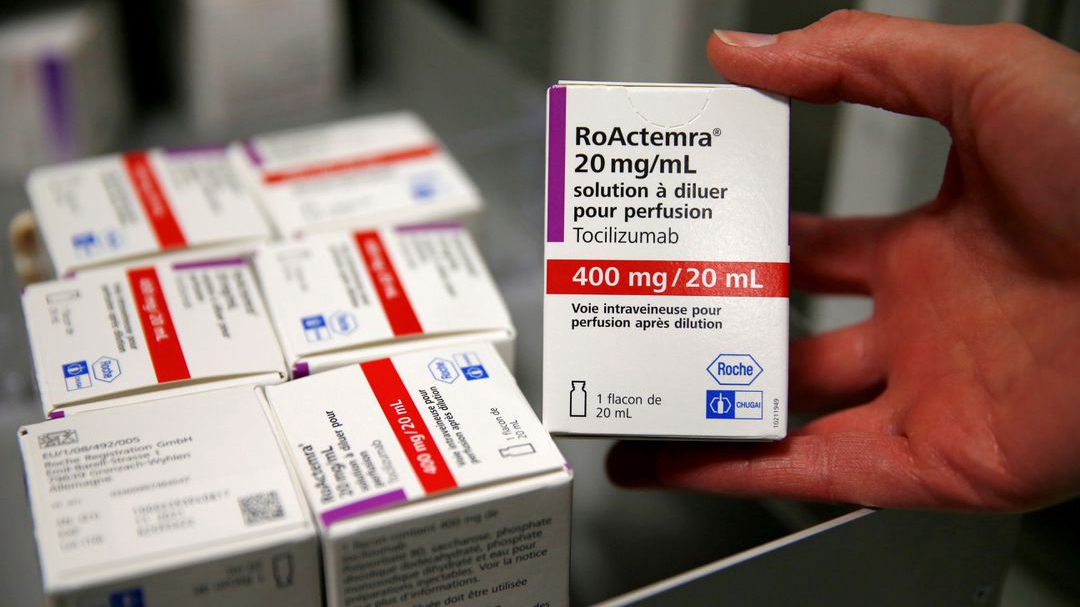
Tocilizumab, which is used in the treatment of rheumatoid arthritis, at a pharmacy in Cambrai Hospital, France, April 28, 2020. /Reuters
In its latest review of a potential coronavirus treatment, Europe's drugs regulator said Monday it was evaluating the use of Roche's arthritis drug, Actemra, in adultshospitalized with severe COVID-19.
Tocilizumab, sold by Roche as Actemra and RoActemra, has shown promise in clinical trials in treating COVID-19, and was approved by U.S. health regulators in June for emergency use in hospitalized COVID-19 patients who needed oxygen.
A large trial in February showed that tocilizumab cut the risk of death among patients hospitalized with severe COVID-19, shortened recovery timeand reduced the need for mechanical ventilation.
The European Medicines Agency (EMA) will carry out an accelerated assessment of the drug, including results from four large studies, it said in a statement. The outcome is expected in mid-October.
The EMA is also evaluating an application for an arthritis drug developed by Sweden's Sobi to treat COVID-19 in adults with pneumonia, and Eli Lilly's rheumatoid arthritis drug Olumiant to treat hospitalized COVID-19 patients receiving oxygen.
Other treatments under a rolling review in the European Union include antibodies or antibody cocktails developed by Eli Lilly, Celltrion and Regeneron, and one jointly developed by GlaxoSmithKline and Vir Biotechnology.
Gilead's remdesivir is the only drug currently authorized to treat COVID-19 in the European Union.
Source(s): Reuters
 简体中文
简体中文