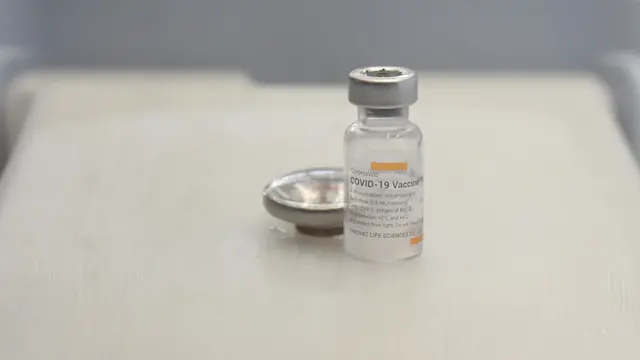
The COVID-19 vaccine developed by China's Sinopharm has been approved for emergency use in the Maldives, the Maldives Food and Drug Administration (MFDA) announced at a press conference Monday afternoon.
The vaccine made by China National Pharmaceutical Group (Sinopharm) will be given to people aged between 18 and 60, local media reported, citing an official at the MFDA.
 简体中文
简体中文