Clinical trials have begun in the UK for a new COVID-19 vaccine being developed in Scotland.
The UK government has pre-ordered 60 million doses of the Valneva candidate, which is being developed at the French biotech company's facility in Livingstone, West Lothian.
It is being tested on 150 volunteers at four National Institute for Health Research (NIHR) testing sites in Birmingham, Bristol, Newcastle and Southampton.
Business Secretary Alok Sharma said he had visited Valneva's "state-of-the art facility" over the summer, adding he had "seen first-hand the incredible work our scientists and researchers are doing" to develop the new vaccine.
How do the Moderna, Pfizer and Oxford coronavirus jab candidates compare?
It comes as the UK rolls out its large-scale vaccination programme of the Pfizer/BioNTech vaccine after becoming the
first country in the world to approve it
on 2 December.
Mr Sharma said: "As we take the monumental steps in rolling out the first
COVID-19
vaccine, we must remember that we need to have a range of vaccines available to protect the British public now and long into the future.
"Today we have more welcome news that life-saving clinical trials will begin across the country to test the safety and effectiveness of Valneva's vaccine, which is being clinically developed right here in the UK."
Initial trials will show if the vaccine produces a safe and effective immune response against COVID-19.
If successful, more tests will be planned for April 2021, with more than 4,000 UK volunteers taking two doses, and the candidate could become available by the end of next year.
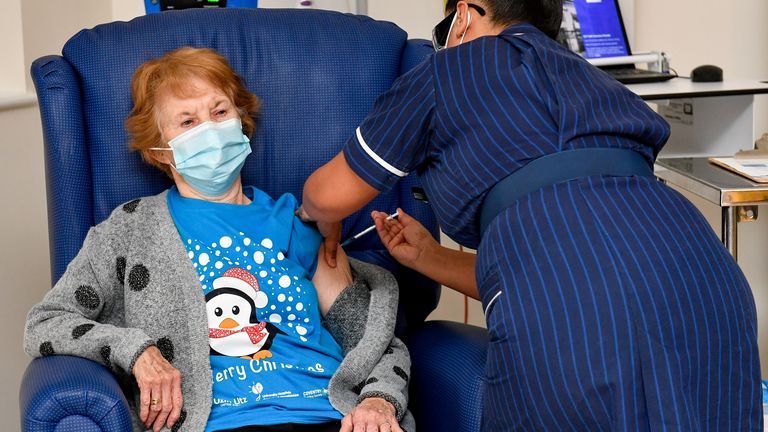
Image:Margaret Keenan became the first patient in the world to receive the Pfizer vaccine outside of a trial
Valneva will potentially have the capacity to supply up to 250 million vaccine doses to the UK and around the world.
The company's chief executive, Thomas Lingelbach, said: "Our teams have been working extremely hard to develop our differentiated vaccine candidate and I would like to thank them, as well as the UK government, for their dedication and support.
How the Pfizer/BioNTech vaccine is being rolled out - and who will get it first
"While conducting our first clinical trials, we are already ramping up our manufacturing capacities and commencing production at full-scale so that we can make the vaccine widely available across the world assuming the vaccine is safe and effective."
Please use Chrome browser for a more accessible video player
GPs in England begin giving COVID vaccines
Last week, grandmother-of-four Margaret Keenan was the
first person in the UK to receive the Pfizer vaccine
outside a trial as people aged 80 and over and care home workers were among the first to receive the jab.
Vaccination centres run by local doctors
in more than 100 locations across England had the Pfizer jab delivered to them on Monday in the latest phase of the rollout, however some scientists are warning it could take at **at least a year to inoculate
** the entire UK population.
 简体中文
简体中文