00:52
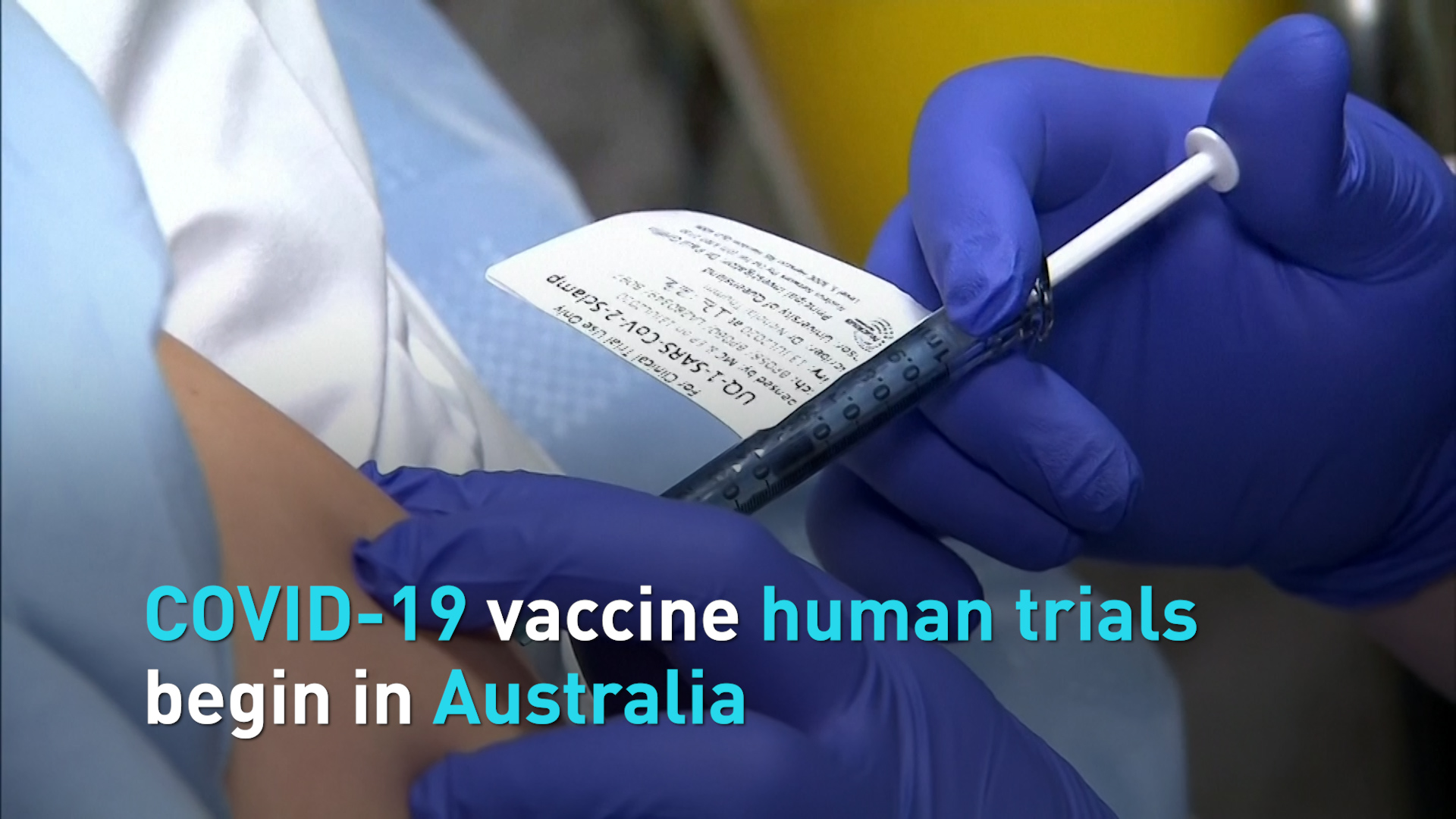
Researchers from the Australian biotech company CSL and the University of Queensland began a Phase I human trial with an experimental COVID-19vaccine on Monday.
They selected 120 test subjected from around 4,000 volunteers. Each will receive two doses of the experimental vaccine - an initial dose followed by a second one after four weeks. Preliminary results could be available by the end of September.
The research team will monitor the volunteers for 12 months. If this experimental vaccine proves safe and effective, and is licensed, itcould be available as early as mid-2021.
In the history of vaccine development, no vaccine has been licensed in less than four years.
In 2003, research began on a vaccine for Severe Acute Respiratory Syndrome (SARS), another coronavirus. Seventeen years later, there is still no vaccine.
Based on a list from the World Health Organization,
The
New York Times reports more than 155 candidate COVID-19 vaccines in development around the world. A total of 23 of them are currently in human trials.
According to John Hopkins University, the global total for COVID-19 cases reached 12,995,037 by 5 p.m. on Monday. The death toll rose to 570,435.
 简体中文
简体中文

