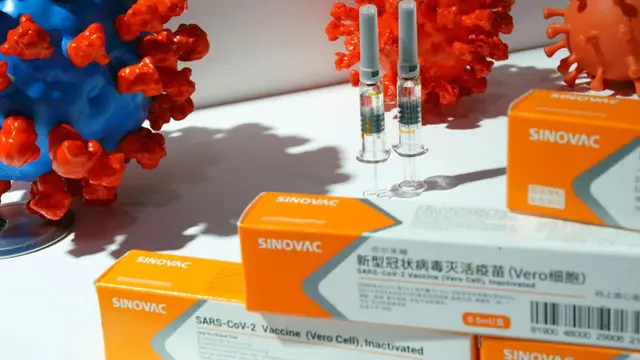
Thailand's Food and Drug Administration (FDA) has given emergency use authorization for Sinovac Biotech's coronavirus vaccine, a senior official said on Monday.
"The FDA has approved or registered Sinovac's COVID-19 vaccine for conditional emergency use, effective February 22," FDA deputy secretary-general Surachok Tangwiwat said, adding it was valid for one year.
 简体中文
简体中文