
Screenshot from the company's website
U.S. Food and Drug Administration (FDA) on Tuesday granted clearance to the first in-home coronavirus test that allows people to collect their own samples at home, a new approach that could help expand testing options in most states.
The test from LabCorp will initially be available to healthcare workers and emergency workers under a doctor's orders. The sample will still have to be shipped for processing back to LabCorp, which operates diagnostic labs throughout the U.S.
Allowing people to self-swab at home would help reduce infection risks for frontline healthcare workers and help conserve protective gear, said the company.
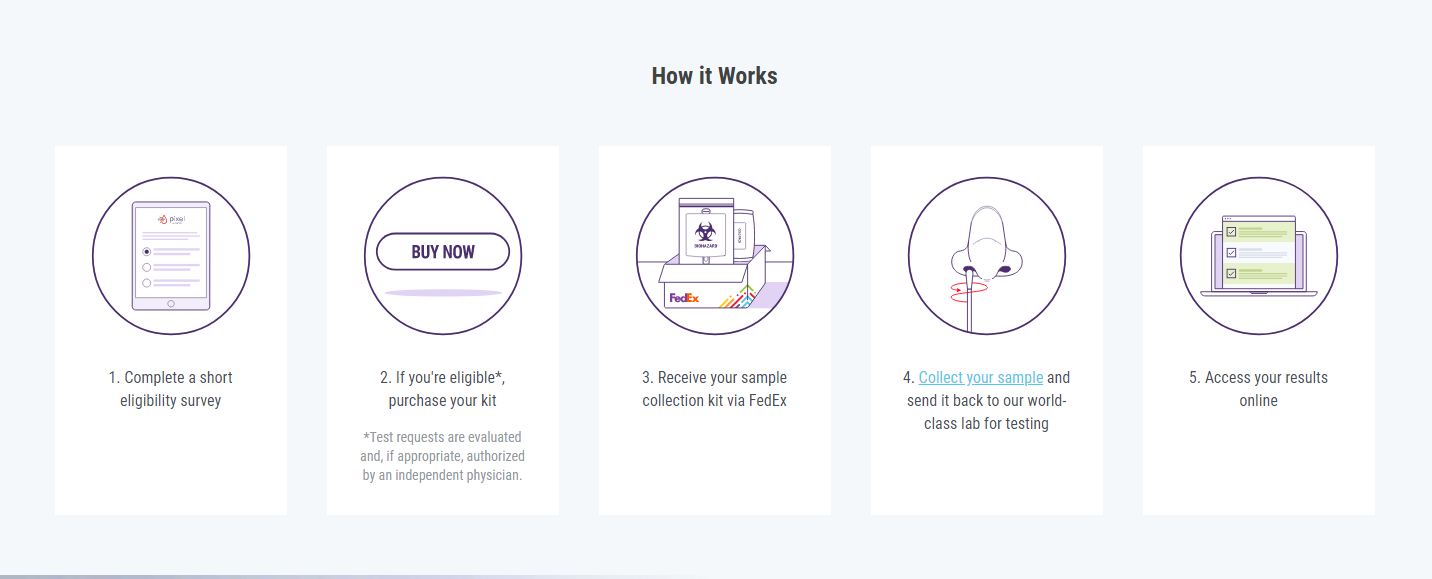
For the home test, people are initially screened with an online questionnaire. If authorized by a physician, LabCorp will ship a testing kit to their residences. The kit includes cotton swabs, a collection tube, an insulated pouch and box to ship the specimen back to LabCorp. To take a sample, a cotton swab is swirled in each nostril. The test results are posted online to a secure company website.
Screenshot from the company's website
The company said it will make the test available in the coming weeks. Each kit will cost 119 U.S. dollars. But customers will have to pay out of their own pocket, the company said, despite the U.S. administration promising to cover the bill for diagnostic tests.
The kits will not be available in Maryland, New Jersey, New York and Rhode Island. Those states have laws prohibiting testing with at-home sample collection kits, according to LabCorp.
"This is not a game-changer, but it's potentially another piece of the large and rather messy puzzle we're assembling to test enough people to stop COVID-19," Dr. Sheldon Campbell, associate director of the Yale School of Medicine's Clinical Microbiology Laboratory, told New York Times. "A home-collection testing procedure will support telemedicine and help patients with transportation difficulties and in hard-to-reach places to access testing."
Specimen collection from different body parts may impact the accuracy of the test results, even though FDA Commissioner Stephen Hahn said in a statement the agency authorized the self-swab test based on data showing it is "as safe and accurate as sample collection at a doctor's office, hospital or other testing site."
A
previous study
conducted by French experts shows nasalsampleshave lower sensitivity to the test kits than those from nasopharyngeal, the part behind the nose and starts in the upper part of your throat which doctors have to go deeper to reach.
Chinese experts have also made similar statements when explaining the "false negative results" in some of the test kits produced in China, saying for nucleic acid testing, the sensitivity of different types of test kits and the operational approach by medical personnel can also cause false positive or negative results in coronavirus testing.
(With input from AP)
 简体中文
简体中文

