
Twelve Chinese medical experts issued a joint paper on Wednesday calling for well-designed and orderly clinical trials on the novel coronavirus (COVID-19) patients.
The
paper
, published on Chinese Journal of Epidemiology, targeted at over 200 clinical trial applications submitted to Chinese Clinical Trial Registry, suggesting more efforts should be put on diagnostic studies and observational studies characterized by clinical symptoms in the current stage.
To put it more clearly, studies on how to diagnose the disease in a fast and accurate way and how to contain the spread and prevent infection should be given priority, rather than the effectiveness of some treatments.

Screenshot from Chinese Journal of Epidemiology
Seventeen years have passed after SARS was officially declared contained by the World Health Organization (WHO) on 5 July 2003, there is still no confirmed treatment that works for every patient. Antiviral medications are sometimes applied to reduce symptoms, but are not effective for everyone. Same thing is likely to go to COVID-19.
The information to the deadly virus is so limited to human beings that it is barely possible for scientists to develop vaccines and confirmed drugs in such an early stage.
Massive clinical trials to evaluate the treatment without rigorous and quality design will increase the burden of frontline medical staff, the paper said. All efforts and collaborations from researchers, patients, and relevant departments will be wasted.
In fact, expert team dispatched by WHO realized the same concern raised by their Chinese peers when they were having a week-long investigation to some Chinese cities including Wuhan, the epicenter of the COVID-19 outbreak.
On Monday's press conference, Bruce Aylward, a Canadian epidemiologist who headed up the international expert mission to China, said there are long lists of research projects and studies across the world.
"But we've got to prioritize those things that could get the knowledge we need to stop this faster and the tools we need to reduce the morbidity and mortality," he added.
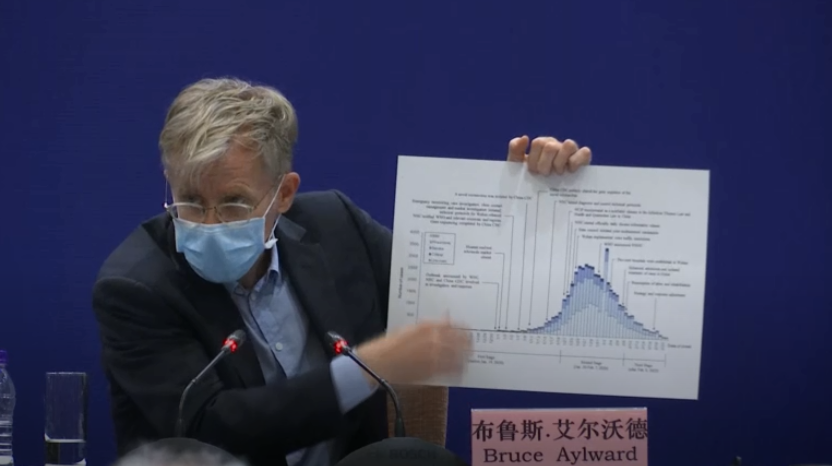
Bruce Aylward, a Canadian epidemiologist who headed a WHO team in China on the coronavirus fight, speaks at a press conference in Beijing, February 24, 2020. /CGTN
He mentioned the research conducted by Cao Bin, director of China-Japan Friendship Hospital and head of the clinical trials of Remdesivir, which is the only drug right now that experts agree may have real efficacy.
Cao said he is having trouble recruiting patients. "It is not just because the numbers are falling, but also because we're doing lots of other studies with things that are less promising."
Aylward stressed that the whole world, not only China, should start prioritizing enrollment into those things that may save lives and save them faster because it's a global issue.
 简体中文
简体中文





