A British company has announced a new type of COVID-19 test that it says can detect people who have contracted the virus using just a saliva swab that can return a result in 15 minutes.
Vatic's KnowNow
COVID-19
test is a type of lateral flow test, but "works in a completely different way to every other antigen test that exists", according to the company's chief executive Alex Sheppard.
Mr Sheppard told Sky News the company was announcing the technological mechanism behind the test ahead of it receiving regulatory approval "simply because it's totally different to anything else out there".

What are the different types of COVID-19 tests?
The test has two crucial differences, the company explained.
Firstly, it uses saliva swabs from the inside of a subject's cheek and under their tongue instead of potentially invasive nasal ones.
Secondly - and what the team says makes it unique - the test reacts to the presence of the spike protein on the outside of the
coronavirus
cell, instead of the nucleocapsid protein within it.
"The chemistry behind the test essentially renders the test as an infectiousness test, because what we're detecting is only the infectious virus - we can't detect non-infectious viruses," Mr Sheppard said.
It is a type of lateral flow test - similar to the nasal swabs already being used. Experts, including scientists at Public Health England's Porton Down laboratory and the University of Oxford, have warned that such tests have too high a false positive rate to replace laboratory ones.
According to Vatic, its test has not produced any false positives during its internal trials - however the company acknowledges a very small sample size of 200 tests, giving at best a 0.5% granular view of its effectiveness.
This is significantly fewer than sample size for the lateral flow test produced by Innova, which was tested on 6,967 people. Of those, 22, or 0.32%, gave false positive results.
Mr Sheppard said that the data available to the team was equivalent to what Innova had at this stage of the development of its product, and expected increased data would show similar or better results regarding false positives.
He added that a clinical trial was beginning in the new year to generate equivalent evidence.
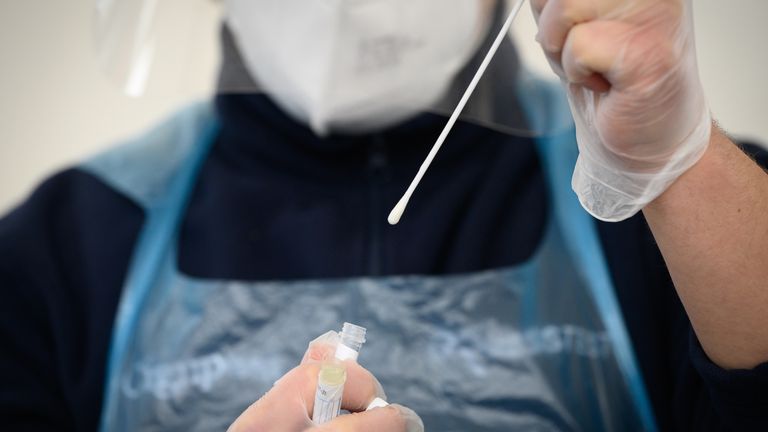
Image:Swab tests have been rolled out at dedicated testing sites, workplaces, airports and elsewhere
The test product, which is about the size of a deck of cards, is currently clearing a number of regulatory hurdles - although it is set to be manufactured in Bedford, "where lateral flow was invented in the early 1980s", as Mr Sheppard notes.
The first of these hurdles is being awarded a CE mark by European regulators, which will have equivalence in UK standards following
Brexit
. After that it can be registered with the UK's MHRA.
"Because the test is initially for professional use, that means that this can be released in that setting faster," he said, adding that studies would follow its release to clinical professionals and enable the company to release the product as a self-test.
"This is a product that is invented, designed, and manufactured in the UK," Mr Sheppard said, adding he did not expect the ongoing Brexit negotiations to impact the company significantly.
 简体中文
简体中文

